Against this backdrop, Quenchbody (Q-body) technology stands out due to its homogeneous "mix-and-read" detection advantages. However, existing Quenchbody technologies suffer from key shortcomings such as the large size and difficult expression of traditional antibodies. To break through these limitations, a research team from the University of Wollongong (Australia) and ESPCI Paris (France), among other institutions, conducted optimization research on nanobody-based Quenchbodies. Their findings were published in Communications Biology. Using molecular dynamics simulations and rational design, the team precisely identified key tryptophan residues in the complementarity-determining regions (CDRs) of nanobodies that regulate fluorescence quenching. They constructed an optimized nanobody scaffold and, through in vitro directed evolution screening, developed a high-performance Quenchbody targeting IL-6—achieving a fluorescence increase of 1.5–2.4 times and a detection limit as low as 1–2 nM. Furthermore, sensors for different protein targets can be rapidly developed within 2–3 weeks, providing a versatile detection platform for clinical diagnostics, environmental monitoring, and other fields.
Mechanism Elucidation: Identifying the Core Role of Tryptophan in Nanobody CDRs
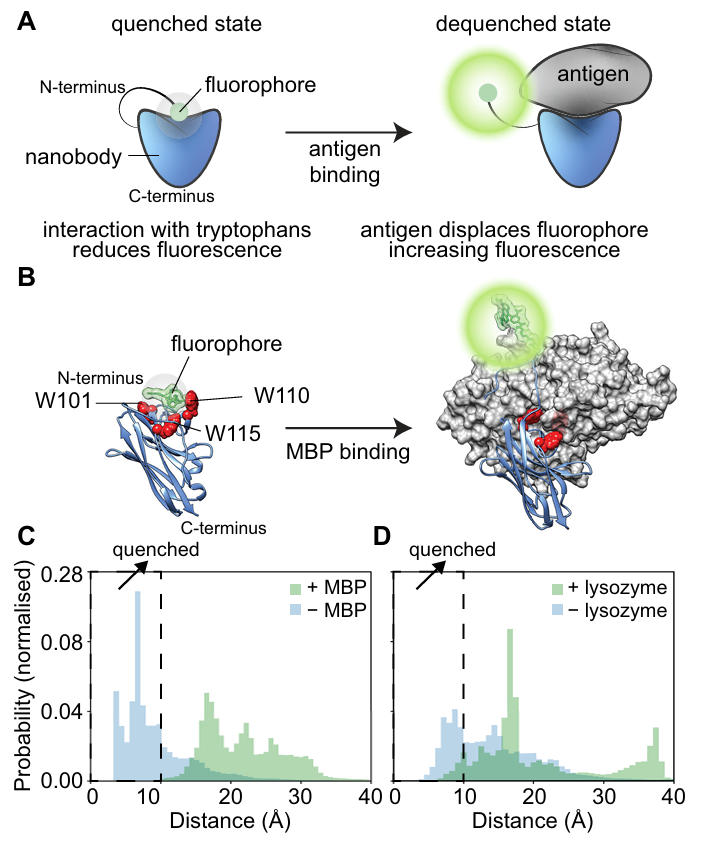
To optimize Quenchbody performance, the team first clarified its fluorescence regulation mechanism. They selected two types of convex paratope nanobodies with high-resolution crystal structures—a maltose-binding protein (MBP)-binder (PDB ID: 5M14) and a lysozyme-binder (PDB ID: 1ZVH)—as research models. Through all-atom molecular dynamics (MD) simulations, they analyzed the interaction between the fluorophore (TAMRA) and nanobody residues.
The simulation results showed that in the absence of antigen, the tryptophan residues in the CDRs of the MBP-binding nanobody (W101, W110, W115) were within ≤10 Å of the fluorophore for 70.7±18.7% of the time, resulting in efficient fluorescence quenching. After binding to the MBP antigen, this proportion dropped sharply to 0.1±0.2%, as the fluorophore was sterically hindered from the tryptophan interaction range, achieving "de-quenching." These results confirm for the first time that tryptophan residues in the CDR regions of nanobodies are the core switch regulating fluorescence quenching and recovery.
Figure 1: Quenchbody working mechanism and molecular simulation results
Scaffold Optimization: Rational Design Enhances Basic Detection Performance
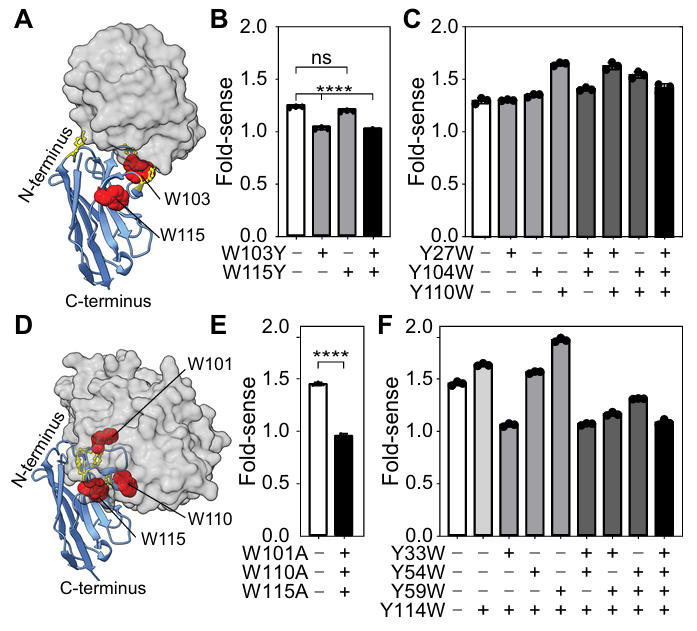
Based on the mechanistic insights, the team validated the function of key tryptophan residues through site-directed mutagenesis and further optimized the scaffold structure. For the lysozyme-binding nanobody, whose CDR region contains three tryptophans (W36, W103, W115), W36 is buried deep inside the β-barrel and cannot contact the fluorophore, while W103 directly binds lysozyme and W115 is only surface-exposed. Mutating W103 to tyrosine (W103Y) significantly reduced the fluorescence increase of the Quenchbody toward lysozyme. Conversely, mutating a tyrosine in the CDR to tryptophan (Y110W) increased the fluorescence enhancement from 1.3-fold (wild-type) to 1.7-fold.
Similarly, for the MBP-binding nanobody, knocking out key CDR tryptophans (W101, W110, W115) caused the Quenchbody to completely lose its fluorescence response. Introducing a Y59W mutation increased the fluorescence enhancement to 1.9-fold. Ultimately, the team constructed a universally optimized scaffold—precisely introducing tryptophan residues in CDR2 (W59) and CDR3 (W101, W103, W110, W115) to form a convexly distributed "quenching center." This resulted in detection limits of 4 nM and 1 nM for the MBP- and lysozyme-targeting Quenchbodies, respectively, with EC50 (half-maximal effective concentration) values of 14 nM and 7 nM. Performance improved by 30%–50% compared to traditional nanobody-based Quenchbodies.
Figure 2: Effect of tryptophan mutations on fluorescence response
Directed Evolution: Developing a High-Performance Detection Tool for IL-6
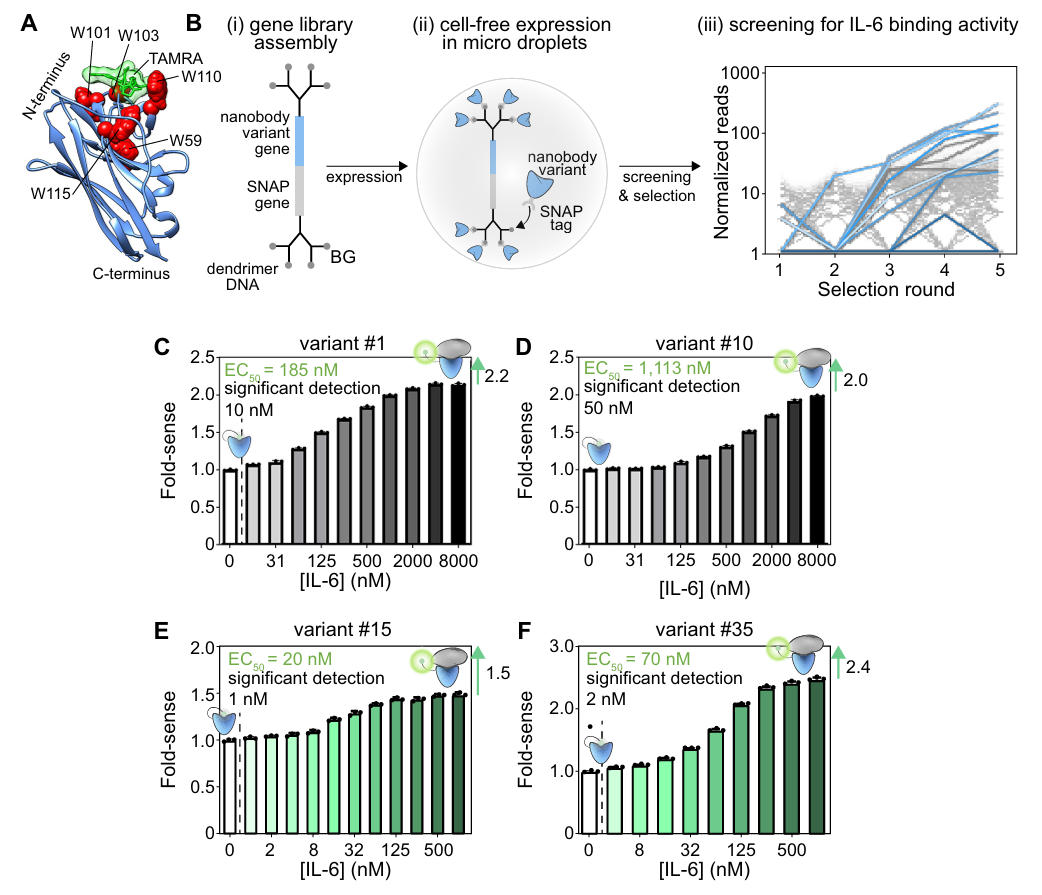
To verify the generality of the scaffold, the team targeted the human inflammatory cytokine IL-6 for in vitro directed evolution screening. First, they randomized the CDR regions of the optimized scaffold using TRIM oligonucleotides to construct a diverse gene library. A SNAP tag was fused to achieve "phenotype-genotype" linkage: each gene variant was linked to dendrimeric DNA containing benzylguanine (BG). After cell-free expression within microdroplets, the SNAP tag covalently bound to BG, ensuring that selected active variants could be traced back to their gene sequences.
After five rounds of magnetic bead screening and NGS sequencing, the team obtained 12 initial positive variants. The best variant, #1, showed a fluorescence increase of 2.2-fold and an EC50 of 185 nM. Using SNAP-display technology, the researchers performed random mutagenesis on the CDR1 and CDR3 regions to construct a nanobody library with randomized CDRs. Through microdroplet encapsulation and in vitro screening, they finally obtained variants #15 and #35. #15 had a detection limit as low as 1 nM and an EC50 of 20 nM; #35 showed a fluorescence increase of 2.4-fold and a detection limit of 2 nM, maintaining stable detection performance even in 50% human plasma. This provides an efficient tool for clinical IL-6 detection.
Figure 3: Directed evolution process and performance of IL-6 Quenchbodies
Throughout this study, nanobodies were not merely detection vehicles but core elements enabling technological breakthroughs. Their unique advantages were evident across the entire process, from mechanism elucidation to application development.
Thanks to their small molecular size and simple structure, nanobodies have CDR regions that are directly exposed on the surface, eliminating the need for indirect fluorophore regulation via VH-VL interfaces. This allows direct interaction between CDR tryptophans and the fluorophore, avoiding the low regulatory efficiency seen in traditional scFv/Fab-based Quenchbodies caused by interfacial allosteric effects. Moreover, the convex binding mode of the selected nanobodies creates significant steric hindrance upon binding protein antigens, effectively pushing the fluorophore away from tryptophan and enabling precise "de-quenching" regulation.
Furthermore, nanobodies exhibit high stability and mutational tolerance, granting them great potential for engineering. The research team significantly enhanced their performance through rational design without worrying about structural misfolding. Additionally, due to their short gene sequences, nanobodies can be easily randomized in their CDRs using TRIM oligonucleotides, enabling the construction of large gene libraries. The entire process—from scaffold construction to target screening—can be completed quickly, far exceeding the efficiency of traditional antibodies.
This study not only provides a high-performance design strategy for nanobody-based Quenchbodies but also demonstrates the powerful potential of combining computer simulations, rational engineering, and directed evolution in modern biosensor development. In the future, with further optimization and industrial advancement, such Quenchbodies are expected to become core tools in the next generation of immunoassays, driving progress in precision medicine, in vitro diagnostics, environmental monitoring, and related fields.