Recently, a groundbreaking study published in the journal Structure has brought new hope to the field of cancer therapy. Conducted jointly by the Department of Pharmacology at the University of Cambridge and the Cancer Research Centre at Newcastle University, this research focuses on the application of nanobodies to restore p16 function. It successfully addresses the challenge of structural instability caused by p16 mutations, paving a new path for targeted cancer therapy.
Construction and Screening of the Anti-p16 Nanobody Library
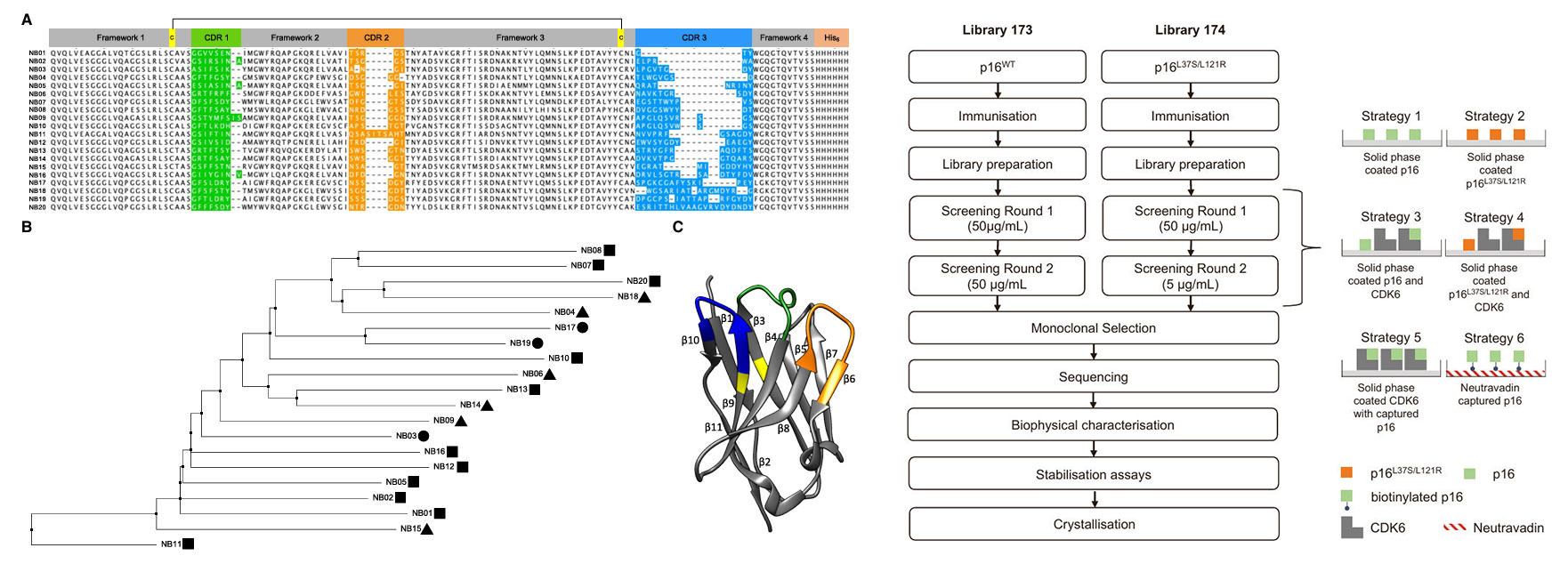
To achieve this goal, the research team began by constructing a high-affinity anti-p16 nanobody library. They optimized the expression and purification of the p16 protein, significantly improving its yield and homogeneity by adding an Avi-tag and introducing stabilizing mutations (W15D, L37S, L121R). Subsequently, llamas were immunized with either wild-type p16 or the double mutant p16L37S/L121R, leading to the construction of two phage display nanobody libraries (Library 173 and 174), with library sizes of 4.2× 1013 and 6.2 × 1013, respectively. Six different biopanning strategies were employed, including solid-phase coated p16, p16-CDK6 complex capture, and streptavidin capture of biotinylated p16. After two rounds of screening, more significant enrichment was observed from Library 174, and unique positive clones were subsequently selected from both libraries across the various strategies. Based on CDR3 sequence alignment, the positive clones were categorized into 6 major families (comprising over 60% of clones) and 16 smaller families. Twenty representative clones were randomly selected for further analysis, 19 of which were expressed efficiently. Phylogenetic tree analysis revealed that these nanobodies had distinct binding epitopes.
Figure 1: Nanobody Families, Structural Analysis & Screening Strategy
Biophysical Characterization of Nanobodies
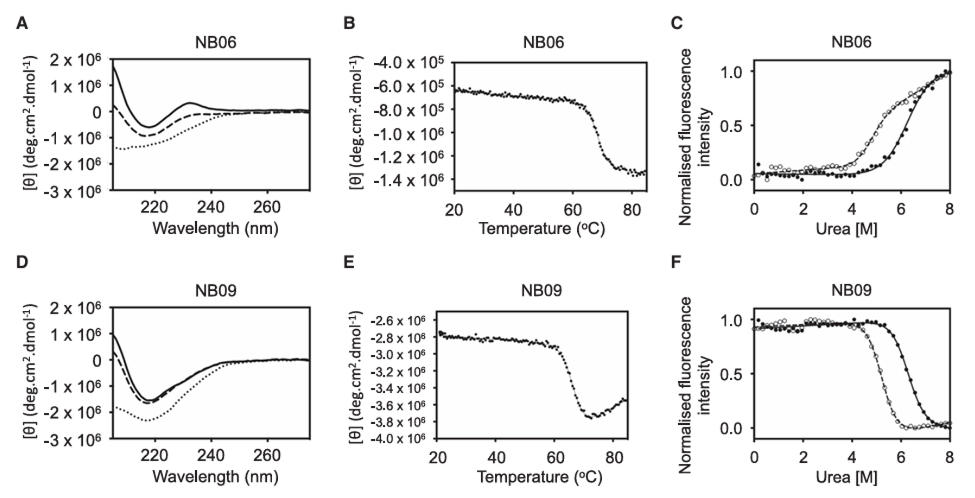
Far-ultraviolet circular dichroism (far-UV CD) analysis showed that all nanobodies exhibited the characteristic antiparallel beta-sheet structure, with a signature minimum at 218 nm, indicating structural integrity. Thermal stability tests revealed that the midpoint of unfolding (Tm) for most nanobodies was between 60-80°C, specifically 68.3°C for NB06 and 66.6°C for NB09. Some nanobodies (e.g., NB04, NB07) demonstrated good refolding capability.
In chemical denaturation experiments, 15 nanobodies displayed cooperative two-state unfolding behavior, with a midpoint of denaturation (D 50%) in the range of 5-7 M urea. Even in the presence of the reducing agent DTT, they remained folded at urea concentrations up to 4 M. Based on comprehensive thermodynamic stability and fluorescence denaturation profiles, six nanobodies—NB03, NB05, NB06, NB09, NB16, and NB17—were selected for functional validation.
Figure 2: Nanobody Characterization
Stabilizing Effect of Nanobodies on p16
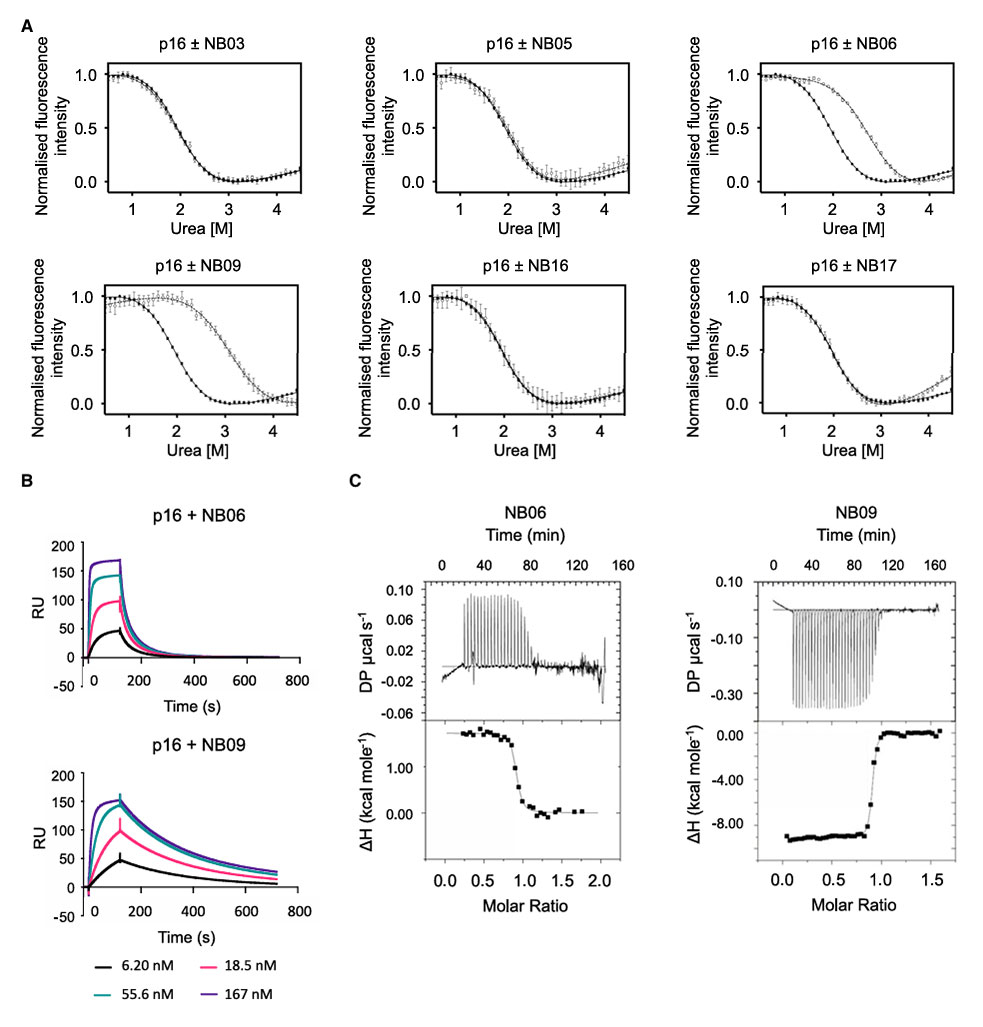
Urea denaturation experiments showed that the D<sub>50%</sub> for wild-type p16 was 1.94 M, which NB06 and NB09 increased to 2.80 M and 3.11 M, respectively. The free energy of unfolding (ΔG<sub>unf</sub>) increased from 3.22 kcal/mol to 4.65 and 5.16 kcal/mol, respectively. The other four nanobodies showed no significant stabilizing effect. Surface plasmon resonance (SPR) and isothermal titration calorimetry (ITC) confirmed that NB09 (K<sub>D</sub> = 7.52 nM) had a higher affinity for p16 than NB06 (K<sub>D</sub> = 19.98 nM), and their binding modes differed (NB06 binding was endothermic, while NB09 binding was exothermic).
Figure 3: Nanobodies stabilize p16
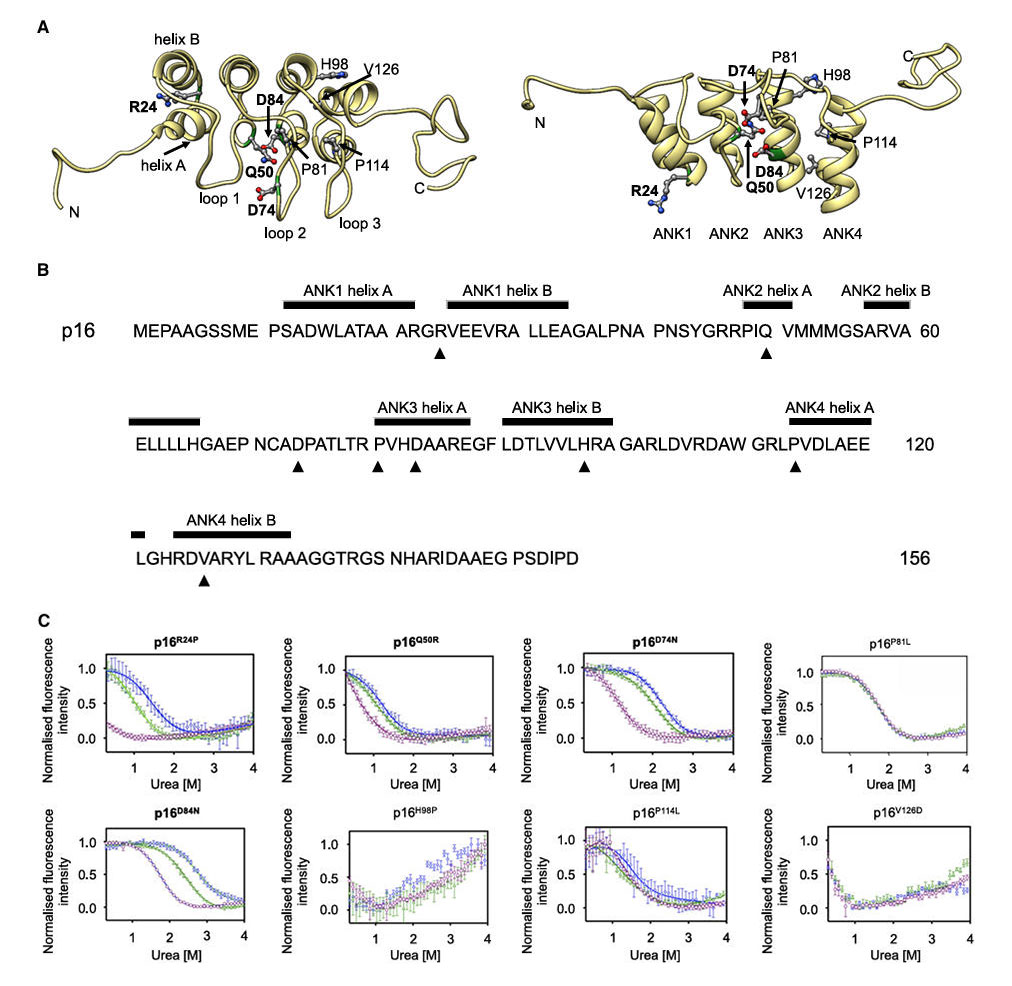
Testing on eight cancer-associated p16 mutants revealed that NB06 and NB09 significantly stabilized the R24P, Q50R, D74N, and D84N mutants. Notably, R24P showed no cooperative unfolding in the absence of nanobodies but regained a typical denaturation curve upon binding. In contrast, the P81L, H98P, P114L, and V126D mutants, likely due to severe structural disruption, were not effectively stabilized.
Figure 4: p16 structure and characterization of cancer-associated mutations
Structural Analysis of the p16-Nanobody Complex
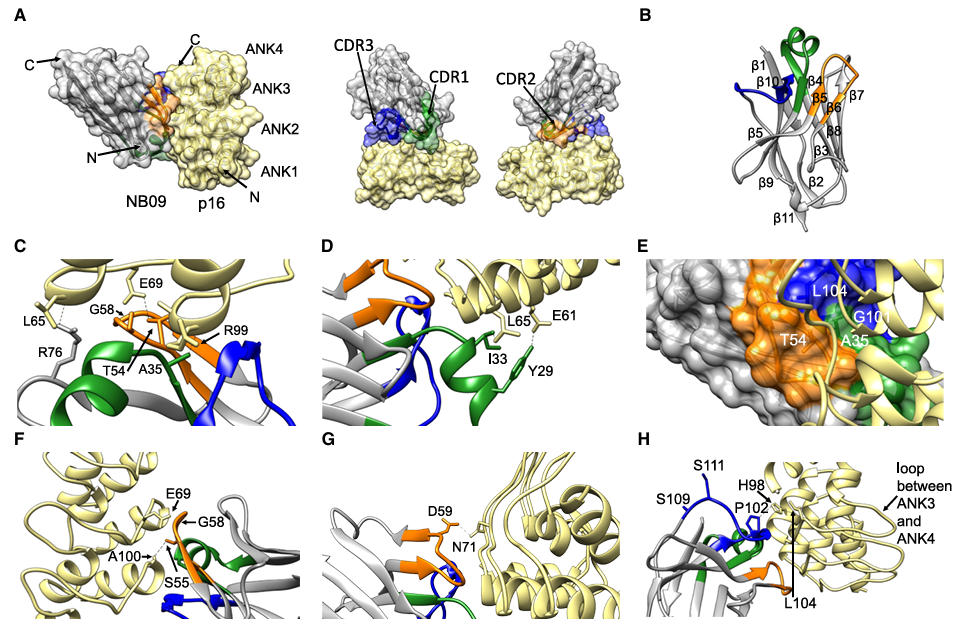
The crystal structure of the p16-NB09 complex was determined at a resolution of 1.79 Å (with the highest resolution shell at 1.74 Å). It revealed that NB09 binds at the interface between ANK2-ANK3 and ANK3-ANK4 of p16, on the face opposite to the CDK-binding site. This binding mode did not significantly alter the overall fold of p16 (RMSD of 0.693 Å compared to the p16-CDK6 complex), laying the foundation for ternary complex formation.
The crystal structure of the p16-NB09 complex was determined at a resolution of 1.79 Å (with the highest resolution shell at 1.74 Å). It revealed that NB09 binds at the interface between ANK2-ANK3 and ANK3-ANK4 of p16, on the face opposite to the CDK-binding site. This binding mode did not significantly alter the overall fold of p16 (RMSD of 0.693 Å compared to the p16-CDK6 complex), laying the foundation for ternary complex formation.
Figure 5: Crystal structure of the p16-NB09 nanobody complex
Ternary Complex Formation and Cellular Functional Validation
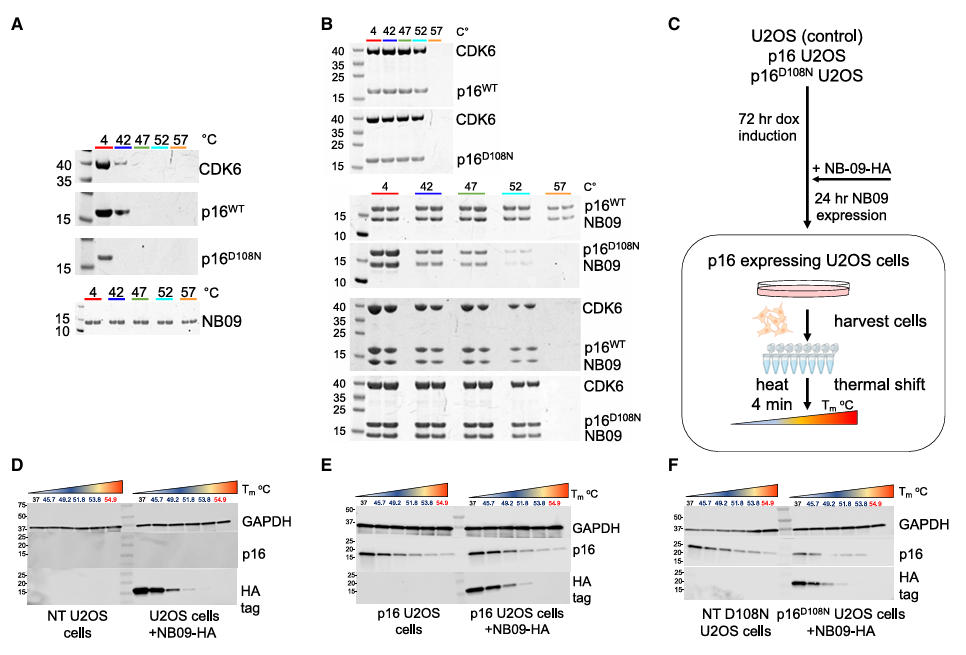
In vitro experiments showed that GST-CDK6 could form a ternary complex with p16 and either NB06 or NB09. Homogeneous time-resolved fluorescence (HTRF) assays confirmed that the nanobodies did not affect the binding affinity between p16 and CDK4/6. Thermal stability tests demonstrated that NB09 could bind the temperature-sensitive mutant p16<sup>D108N</sup>, and the resulting ternary complex (CDK6-p16<sup>D108N</sup>-NB09) remained detectable at 52°C, confirming its stabilizing role within this complex system.
In cellular experiments, HA-tagged NB09 bound endogenous p16 in HEK293T cells and, via p16, co-precipitated CDK4 and CDK6. In U2OS cells with inducible p16 expression, NB09 binding did not interfere with the ability of p16 to induce G1 phase arrest, confirming that it maintains p16 activity within cells.
Figure 6: Characterization of CDK6-p16-nanobody complexes
Empowering Advantages of Nanobodies
Nanobodies offer high affinity and specificity, enabling nanomolar-level binding to p16. NB09, for instance, forms stable interactions with p16 via an alpha-helix in CDR1 and a hydrogen-bond network involving CDR2 and CDR3, increasing the stability of wild-type p16 by over 60%. Crucially, its binding site avoids the CDK interaction surface, allowing it to stabilize the structure while preserving p16 function—a level of precision difficult to achieve with traditional small-molecule inhibitors. Their superior structural stability and penetrating ability allow them to remain folded under high temperature, high urea concentrations, and reducing environments. Their small size not only facilitates crystallographic studies but also promises better penetration into dense tumor tissue, advantageous for future in vivo applications.
The crystal structure confirms that nanobody binding does not alter the functional conformation of p16. Cellular experiments further verify that nanobodies can coexist with the p16-CDK complex without disrupting cell cycle regulation. This "stabilization without interference" characteristic overcomes the potential problem of traditional chaperones affecting normal protein function. Unlike tools targeting specific mutations, NB06 and NB09 can stabilize various mutants located in different regions of p16, including solvent-exposed (R24P, Q50R) and helix-buried (D74N, D84N) mutations. This demonstrates their potential to address multiple common p16 inactivation mutation types, indicating broad application prospects.
This study successfully identified 20 anti-p16 nanobodies, among which NB06 and NB09 bind p16 with nanomolar affinity, significantly enhancing the structural stability of both wild-type and several cancer-associated mutants. The resolved crystal structure of the p16-NB09 complex shows that the nanobody binds on the face opposite to the CDK-binding site, enabling the formation of a ternary complex without interfering with p16-CDK6 interaction. Cellular experiments confirm that nanobodies can bind p16 intracellularly and maintain its function, laying the foundation for developing p16-targeting pharmacological chaperones.

Wuhan Nano Body Life Science and Technology Co. Ltd. (NBLST) is a nanobody industry platform established under the initiative of the Wuhan Industrial Innovation and Development Research Institute. Its headquarters is located in the main building of the Wuhan Industrial Innovation and Development Research Institute in the East Lake High-tech Development Zone, Wuhan. It boasts a 1400 m² independent laboratory in the Precision Medicine Industrial Base of Wuhan Biolake. Additionally, NBLST has established alpaca experimental and transfer bases in Zuoling, Wuhan, and Tuanfeng, Huanggang, both compliant with laboratory animal standards. These bases currently house over 600 alpacas, providing "zero-immunization-background" guaranteed alpaca immunization services for research institutions and antibody drug development companies.
NBLST focuses on the development, engineering, and application of nanobodies, and is dedicated to building an integrated public experimental service platform for production, education, and research. It possesses a full-chain technology platform encompassing antigen preparation (peptides, proteins, and RNA), antibody discovery and engineering, through to biological function validation/screening. The RNA antigens include RNA structurally and sequentially optimized for alpacas. Antibody discovery and engineering services employ multiple technological routes, including phage display, RNA, and mammalian cell display. Through cross-complementation of multiple platforms, it provides flexible antibody discovery and engineering services for pharmaceutical companies and research institutes, facilitating the development of drug reagents.
In addition to its natural nanobody library, NBLST also offers an off-the-shelf immunized library to help clients quickly screen for antibody molecules that meet their needs.
If you require our services, please feel free to contact us via email: marketingdept@nanobodylife.com